Retinopathy of prematurity (ROP) is a vaso-proliferative retinal disease in premature infants and is a leading cause of childhood blindness worldwide.1 High-income countries like the United States have relatively advanced systems for ROP screening in place, including a well-trained workforce and health care resources and infrastructure. However, in regions with limited resources or a shortage of trained ophthalmologists who can screen for and treat ROP, there may be delayed diagnoses, more severe disease, or vision loss. About 65% of ROP cases occur in low-income and middle-income countries (LMICs), where limited healthcare resources and a shortage of trained ophthalmologists can delay diagnosis and treatment.2 Screening for ROP typically requires specialized equipment and trained staff that may be inaccessible in these underserved regions.
Even in well-resourced countries, the diagnosis of ROP is often subjective and can vary significantly between clinicians. Studies of expert diagnosis of ROP have demonstrated large variability in expert diagnosis of plus disease or other clinically significant disease.3,4 Interexpert variability has also been reported across different regions.5 This variability has implications for the management of ROP and can lead to clinically significant differences in outcomes, suggesting the need for further standardization.
Advances in imaging and artificial intelligence (AI) systems offer potential opportunities to improve objectivity and standardization of ROP diagnosis, and expand remote disease detection, monitor progression, and predict risk.6 However, implementation of AI systems in clinical practice, particularly in diverse global settings, may be challenging. Regulatory considerations, infrastructure requirements, and integration into existing workflows are needed to ensure successful implementation.
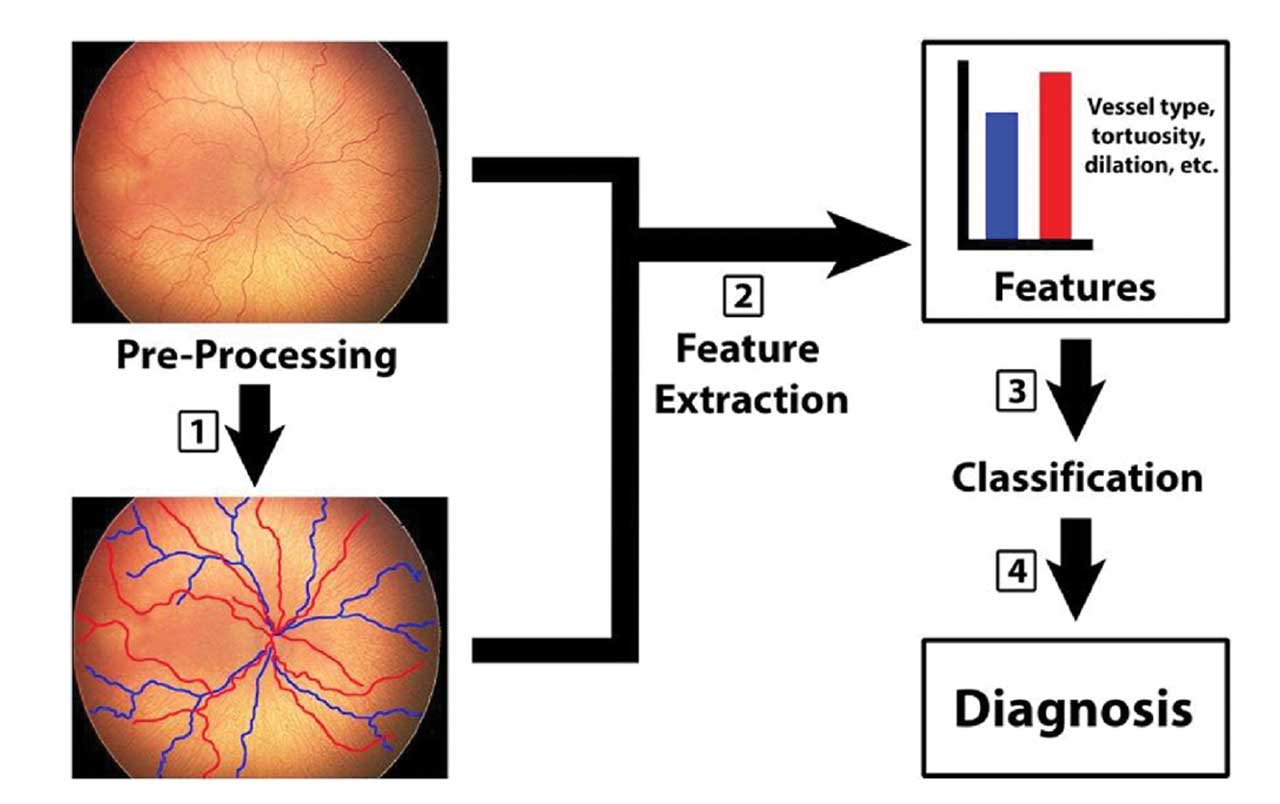
Figure 1. Machine learning in retinopathy of prematurity (ROP). Early attempts at quantifying vascular changes in ROP used manually defined features—such as dilation and tortuosity (step 1 and 2)—with no computer-based classification. With machine learning, classifiers (eg, support vector machines) learn relationships (step 3) between such features and diagnosis (step 4). By contrast, deep convolutional neural networks (CNNs) can learn relevant features directly from input images (step 1), with or without preprocessing, bypassing explicit human-defined features (step 2). Adapted with permission from: Scruggs BA, Chan RVP, Kalpathy-Cramer J, Chiang MF, Campbell JP. Artificial intelligence in retinopathy of prematurity diagnosis. Transl Vis Sci Technol. 2020;9(2):5. doi:10.1167/tvst.9.2.5. Licensed under CC BY-NC-ND 4.0.
Current Technologies
Early computer-based systems for ROP diagnosis—such as ROPTool, Retinal Image multiScale Analysis, Vessel Map, and Computer Assisted Image Analysis of the Retina—used algorithms to semiautomatically measure blood vessel tortuosity and dilation.7 However, unlike current machine learning (ML) and deep learning (DL) tools, these systems did not use adaptive computer learning abilities. Figure 1 depicts the process of ML in ROP.
The Imaging and Informatics in ROP (i-ROP) DL system, developed and maintained by the i-ROP consortium, introduced the concept of an ROP vascular severity score (VSS) and is being developed both as an assistive diagnostic device and as an autonomous screening tool for “more than mild ROP” (MTM-ROP; defined as type 2 or preplus or worse). In a pilot study of the assistive diagnostic functionality, use of an AI-derived VSS was associated with more precise and accurate plus disease diagnosis among ROP clinicians.8 When used fully autonomously, with no human interpretation or preprocessing required, the system performed with 100% sensitivity for detection of MTM-ROP in 2 large real world telemedicine programs, in the United States and India.9 Although there are regulatory and medicolegal challenges to autonomous ROP implementation, autonomous ROP screening could dramatically reduce the workload for the ROP grader in a telemedicine workflow and provide real-time diagnosis to the family and care team.
Other systems have been developed to not only diagnose plus disease but also grade ROP severity or classify zone or stage specifically. Shenzhen Eye Hospital in China developed a DL algorithm that can automatically identify the border of zone 1 on a fundus image to use as a diagnostic aid.10 This group also developed an AI system that identifies disease, identifies aggressive disease, and recommends treatment options for ROP. This AI system achieved an accuracy of 92% in identifying ROP, identifying aggressive disease, and suggesting treatment modalities, outperforming 4 experienced ophthalmologists.11 Another algorithm developed in India demonstrated high sensitivity and specificity for detecting the presence of ROP using images from a tele-screening program.12
Validation and Workflow Integration
Although AI systems have the potential to transform ROP care, successful implementation of AI requires clinical validation across diverse patient populations, imaging systems, and clinical settings. This requires being trained on large, diverse data sets that reflect variability in disease severity, patient demographics, image quality, and the types of cameras used to capture images. Systems that perform successfully in high-income countries or high-resource areas may not perform as well in LMICs, due to these variations.13 Expanding training data sets and testing AI systems across different imaging devices, clinical settings, and global regions can support progress toward clinical validity and generalizability.
Integration into existing infrastructure and workflows is equally necessary to ensure effective implementation. In remote regions, AI systems are often incorporated into telemedicine platforms.2 However, this integration may require reliable internet or Wi-Fi infrastructure that can ensure that trained clinicians can capture images reliably and that at-risk infants can obtain regular, long-term follow-up. Effective training for ophthalmologists and clinicians in ROP diagnosis and management is needed to ensure that they can accurately capture images, interpret AI-generated results, and provide the necessary care and treatment. Educational efforts must be tailored to the specific resources available in different regions.
Global Health Implications
AI has the potential to reduce disparities in ROP care by providing diagnostic screening tools to regions with limited health care resources or infrastructure. However, the use of AI is not the same for every region, and implementation should be adapted to local needs. Some regions may benefit from assistive AI, in which the technology supports clinicians by helping them make diagnoses more efficiently and accurately, while the clinician retains final decision-making authority. Other regions may benefit from autonomous AI, in which the technology itself provides the diagnosis, with subsequent intervention and follow-up carried out by a trained clinician.
AI systems can analyze retinal images with efficiency and offer diagnostic support in areas where expert ophthalmologists are not readily available. They may also improve diagnostic accuracy and enable more consistent diagnoses, which is useful across all regions, especially in those with fewer ROP experts. AI systems can also be integrated into telemedicine platforms, allowing for remote screening and diagnosis in areas where access to specialists is limited.2,13
Challenges to Implementation of AI
There remain challenges to implementing AI systems into clinical practice. One of the largest challenges is need for large, diverse training data sets to ensure unbiased and generalizable performance.14 There are also ethical factors to consider, including data security, privacy, and liability.
Regulatory pathways differ widely by country or region, and alignment with local approval processes is necessary to ensure safe, ethical, and effective medical care. In the United States, regulatory bodies such as the US Food and Drug Administration oversee the approval of AI-based medical devices. However, different countries and institutions may have different regulatory frameworks and standards for the use of AI systems in clinical care, and navigating the regulatory requirements can be a source of delay in deployment globally.
Training for local staff and clinicians is also critical. Introducing new AI-based technologies requires technical understanding, and successful implementation will depend on educating providers to ensure they are able to use these new tools effectively in their existing workflows.
Implementing AI-Assisted ROP Screening
We are currently working with Orbis Mongolia to evaluate the potential for AI-based ROP screening for infants in Mongolia. Our preliminary data demonstrated that the AI system developed by the i-ROP consortium can diagnose severe ROP in retrospective evaluation of data from India, Nepal, and Mongolia.15 Building on this, we are now implementing a program to assess the AI system performance and identify operational challenges to implementation.
Key challenges include equipment logistics and maintenance, imaging quality, workforce training, and clinical workflow integration. These issues are further exacerbated in resource-limited settings, where both infrastructure and staff may be insufficient. There is a need for ongoing training and support during implementation of new programs. Integrating new technology into the daily workflow of clinicians requires careful planning and flexibility and consideration of unique needs and practices. These challenges highlight that the successful implementation of AI screening depends on the consideration of existing infrastructure and creating sustainable workflows.
Future Directions and Conclusion
AI systems have the potential to enhance ROP care by increasing access globally and improving diagnostic accuracy and consistency. Future AI systems must be trained on larger, more diverse data sets to improve their ability to identify and classify ROP across populations and settings.
For AI to be effective in clinical practice, it must be integrated into existing clinical workflows and infrastructure. This includes training personnel, addressing equipment logistics, and providing ongoing support for local clinicians. These strategies should be tailored to local needs and resources to ensure long-term sustainability.
Education and training may be tools to support the use of AI in ROP care. Tele-education has been shown to improve diagnostic accuracy for ROP in both high-resource and low-resource settings.16,17 These platforms can be used to expand the ROP workforce in underserved regions by training clinicians in effective ROP diagnosis and management.
Overall, there has been significant progress in the use of AI in ROP care during the past decade, and advances in AI may assist in providing access to ROP care globally. Moving forward, further efforts are needed to validate AI systems in diverse patient populations and clinical settings and to train clinicians on effective use of these technologies. Through global collaboration, AI has the potential to make ROP care more equitable and accessible worldwide. RP
References
1. Kim SJ, Port AD, Swan R, Campbell JP, Chan RVP, Chiang MF. Retinopathy of prematurity: a review of risk factors and their clinical significance. Surv Ophthalmol. 2018;63(5):618-637. doi:10.1016/j.survophthal.2018.04.002
2. Tsai ASH, Yip M, Song A, et al. Implementation of artificial intelligence in retinopathy of prematurity care: challenges and opportunities. Int Ophthalmol Clin. 2024;64(4):9-14. doi:10.1097/iio.0000000000000532
3. Chiang MF. Interexpert agreement of plus disease diagnosis in retinopathy of prematurity. Arch Ophthalmol. 2007;125(7):875. doi:10.1001/archopht.125.7.875
4. Campbell JP, Ryan MC, Lore E, et al. Diagnostic discrepancies in retinopathy of prematurity classification. Ophthalmology. 2016;123(8):1795-1801. doi:10.1016/j.ophtha.2016.04.035
5. BOOST II Retinal Image Digital Analysis (RIDA) Group, Fleck BW, Williams C, et al. An international comparison of retinopathy of prematurity grading performance within the Benefits of Oxygen Saturation Targeting II trials. Eye (Lond). 2018;32(1):74-80. doi:10.1038/eye.2017.150
6. Valikodath NG, Cole E, Chiang MF, Campbell JP, Chan RVP. Imaging in retinopathy of prematurity. Asia Pac J Ophthalmol (Phila). 2019;8(2):178-186. doi:10.22608/APO.201963
7. Wittenberg LA, Jonsson NJ, Paul Chan RV, Chiang MF. Computer-based image analysis for plus disease diagnosis in retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2012;49(1):11-19. doi:10.3928/01913913-20110222-01
8. Coyner AS, Young BK, Ostmo SR, et al. Use of an artificial intelligence–generated vascular severity score improved plus disease diagnosis in retinopathy of prematurity. Ophthalmology. 2024;131(11):1290-1296. doi:10.1016/j.ophtha.2024.06.006
9. Coyner AS, Murickan T, Oh MA, et al. Multinational external validation of autonomous retinopathy of prematurity screening. JAMA Ophthalmol. 2024;142(4):327-335. doi:10.1001/jamaophthalmol.2024.0045
10. Zhao J, Lei B, Wu Z, et al. A deep learning framework for identifying zone I in RetCam images. IEEE Access. 2019;7:103530-103537. doi:10.1109/access.2019.2930120
11. Liu Y, Du Y, Wang X, et al. An artificial intelligence system for screening and recommending the treatment modalities for retinopathy of prematurity. Asia Pac J Ophthalmol (Phila). 2023;12(5):468-476. doi:10.1097/apo.0000000000000638
12. Rao DP, Savoy FM, Tan JZE, et al. Development and validation of an artificial intelligence–based screening tool for detection of retinopathy of prematurity in a South Indian population. Front Pediatr. 2023;11:1197237. doi:10.3389/fped.2023.1197237
13. Scruggs BA, Chan RVP, Kalpathy-Cramer J, Chiang MF, Campbell JP. Artificial intelligence in retinopathy of prematurity diagnosis. Trans Vis Sci Tech. 2020;9(2):5. doi:10.1167/tvst.9.2.5
14. Gensure RH, Chiang MF, Campbell JP. Artificial intelligence for retinopathy of prematurity. Curr Opin Ophthalmol. 2020;31(5):312-317. doi:10.1097/icu.0000000000000680
15. Cole E, Valikodath NG, Al-Khaled T, et al. Evaluation of an artificial intelligence system for retinopathy of prematurity screening in Nepal and Mongolia. Ophthalmol Sci. 2022;2(4):100165. doi:10.1016/j.xops.2022.100165
16. Campbell JP, Swan R, Jonas K, et al. Implementation and evaluation of a tele-education system for the diagnosis of ophthalmic disease by international trainees. AMIA Annu Symp Proc. 2015;2015:366-375.
17. Chan RVP, Patel SN, Ryan MC, et al. The Global Education Network for Retinopathy of Prematurity (Gen-Rop): development, implementation, and evaluation of a novel tele-education system (an American Ophthalmological Society thesis). Trans Am Ophthalmol Soc. 2015;113:T2.













