Interleukin-6 (IL-6) is a pleiotropic cytokine that is produced by a variety of cell types, including monocytes, macrophages, T-lymphocytes, and synovial fibroblasts.1,2 In this review, we discuss the mechanism of action of IL-6 in proinflammatory signaling pathways, the role of IL-6 as a therapeutic target, and the current IL-6 inhibitor therapies that are available or currently under investigation for noninfectious uveitis (NIU).
Mechanism of Action of IL-6
IL-6 is a 4-helix bundle proinflammatory cytokine that is stimulated during the inflammatory response from tissue injury or infection.3 Stressed or injured cells produce pathogen-associated molecular patterns (PAMPs) from pathogens or damage-associated molecular patterns (DAMPs).4,5 DAMPs and PAMPs are recognized by pattern recognition receptors on immune cells, which in turn stimulate toll-like receptors (TLRs) to activate signaling pathways that produce IL-6 along with other inflammatory cytokines, including TNF-α and IL-1β.6,7

Figure 1. The IL-6 classical and trans-signaling pathways with targets for monoclonal antibodies depicted. Adapted from Serizawa K, Tomizawa-Shinohara H, Miyake S, Yogo K, Matsumoto Y. Interleukin-6: evolving role in the management of neuropathic pain in neuroimmunological disorders. Inflamm Regen. 2021;41(1):34. doi:10.1186/s41232-021-00184-5. Material is available under Public Creative Commons License: https://creativecommons.org/licenses/by/4.0/.
IL-6 functions chiefly through 2 different proinflammatory cellular pathways: the classical pathway and trans-pathway.8,9 In the classical pathway, IL-6 binds to the membrane-bound type I IL-6 receptor (mIL-6R) complex, which then binds with the membrane glycoprotein gp130. In the trans-pathway, IL-6 binds to a soluble IL-6 receptor (sIL-6R), which subsequently binds with gp130. The gp130 homodimer serves as a common junction between the classical and trans-pathways11(Figure 1) and prompts the initiation of intracellular signaling by activating the Janus kinase family tyrosine kinases (JAKs).11,12 Activated JAKs lead to phosphorylation and activation of signal transducers and activators of transcription 3 (STAT3).12 Once STAT3 is phosphorylated, it can translocate to the nucleus and regulate the transcription of several proinflammatory genes.
The IL-6 signaling pathway has several roles in promoting the proinflammatory state including promoting the differentiation of CD4-positive T helper (Th) cells into Th17 cells13 and differentiation of CD8-positive cells into cytotoxic T-cells.14 Therapeutic agents targeting IL-6 achieve their effects via several principal pathways: directly inhibiting either the IL-6, the mIL-6R or sIL-6R, the gp130, or the downstream kinase or transcription factors in the JAK-STAT pathway. These pathways have been studied as potential targets for monoclonal antibody therapies in a broad range of immunologic conditions including NIU. Figure 1 also shows therapeutic targets for inhibition in the IL-6 signaling pathway.
Discussion of Current Therapies
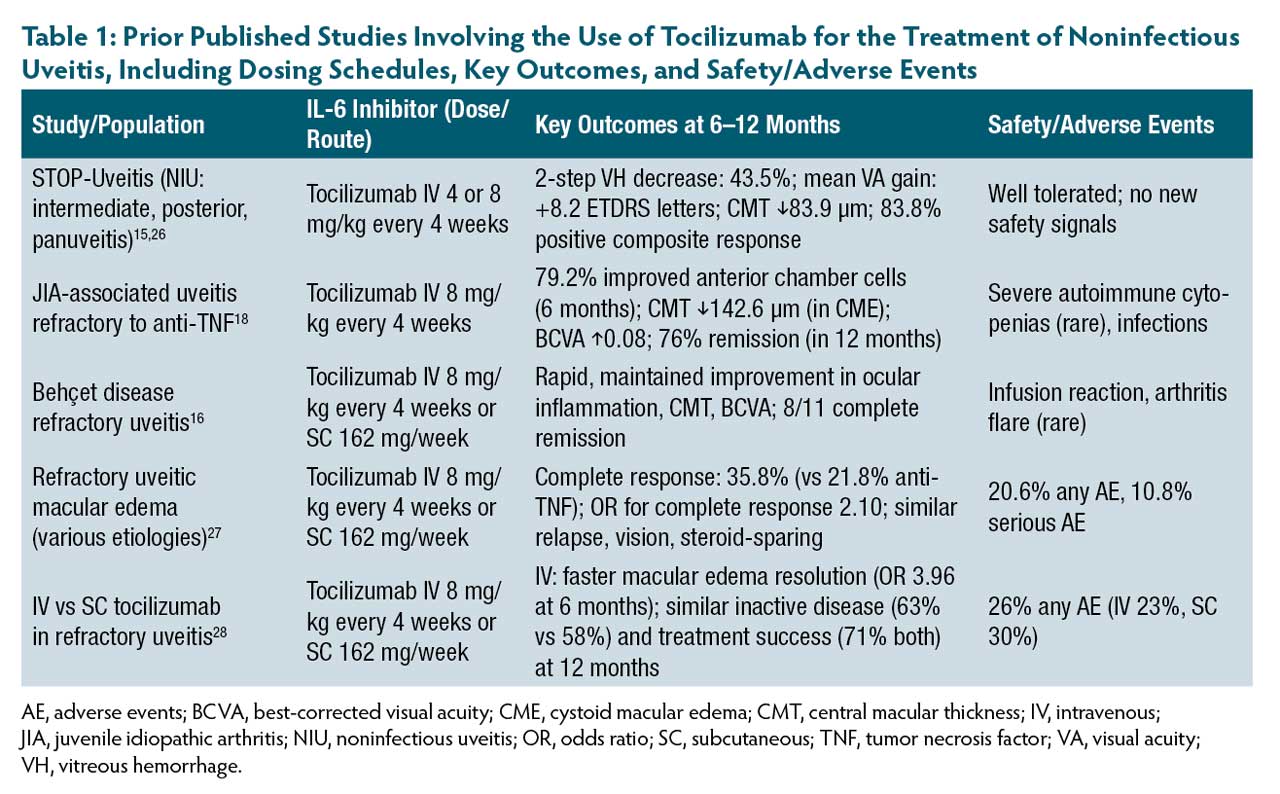
Tocilizumab (Genentech) is a monoclonal antibody against mIL-6R and sIL-6R. The US Food and Drug Administration (FDA) has approved this antibody for the treatment of moderate-to-severe active rheumatoid arthritis (RA) refractory to 1 or more disease-modifying biologics and active polyarticular and systemic juvenile idiopathic arthritis (JIA). Tocilizumab has demonstrated efficacy in the treatment of refractory NIU15-18 and uveitis-associated cystoid macular edema (CME).19 We have observed similar efficacy in our clinical practice (Figure 2).

Figure 2. A 21-year-old female with intermediate uveitis with retinal vasculitis and cystoid macular edema of the right eye as seen on fundus photo, late-phase fluorescein angiography (FA), and optical coherence tomography (OCT), respectively. The patient was initially nonresponsive to a combination of an antimetabolite (methotrexate) and anti-TNF treatment (adalimumab 40 mg every week) (A). She had an excellent response when switched from adalimumab to intravenous tocilizumab 680 mg monthly, with resolution of angiographic leak on late-phase FA and cystoid macular edema on OCT 8 weeks after the switch (B).
STOP-Uveitis was a multicenter, randomized, open-label clinical trial that investigated the role of tocilizumab in 37 patients with NIU. Patients were randomized to receive either 4 mg/kg or 8 mg/kg tocilizumab as an intravenous (IV) infusion. Patients receiving tocilizumab were found to have significant improvement in best-corrected visual acuity (BCVA) and reduction in central macular thickness and vitreous haze in both groups after 6 months.15
A multicenter trial of 25 patients with JIA-associated uveitis refractory to anti–tumor necrosis factor (anti-TNF) therapy receiving IV 8 mg/kg tocilizumab every 4 weeks showed improved BCVA, anterior chamber cell, and central macular thickness.18 Another retrospective study examined 17 patients with JIA-associated uveitis refractory to previous topical and systemic corticosteroids, methotrexate, and biologics including anti-TNF, who were subsequently treated with tocilizumab and demonstrated significantly improved central macular thickness.20 Table 1 outlines key published studies involving the use of tocilizumab for the treatment of NIU, including dosing schedules, key outcomes, and safety/adverse events.
Sarilumab (Regeneron) is a human anti-IL-6 receptor monoclonal antibody that is FDA-approved for the treatment of refractory moderate-to-severe active RA. The SATURN study was a randomized, double-masked, placebo-controlled phase 2 study of patients with non-infectious intermediate, posterior, or panuveitis receiving either subcutaneous sarilumab 200 mg or placebo with a primary endpoint of a ≥2-step reduction in vitreous haze or with a reduction of systemic corticosteroids to a dose of <10 mg/day at week 16. The study found that in eyes with vitreous haze grade ≥2 at baseline, there was a significant decrease in vitreous haze and reduction in systemic corticosteroids in the sarilumab group along with greater mean BCVA gain compared to placebo.21 We have shown resolution of CME and improvement in perivascular leakage on FA associated with autoimmune retinopathy with sarilumab.22 Our group has also demonstrated that tocilizumab or sarilumab was effective in reducing macular edema, reconstituting the ellipsoid zone on OCT, and improving BCVA in patients with autoimmune retinopathy with macular edema.23
Olokizumab (UCB), clazakizumab (Alder Bio- Pharmaceuticals) and sirukumab (Janssen Biologics/Glaxo SmithKline) are other humanized anti-IL-6 monoclonal antibodies currently under investigation for the treatment of RA.
Vamikibart (Roche) is a recombinant, humanized, monoclonal antibody made for intravitreal delivery that binds to and inhibits IL-6 activity. It is currently under investigation for the treatment of uveitic macular edema (UME). MEERKAT and SANDCAT are currently 2 identical ongoing global, randomized, double-masked, sham comparator–controlled phase 3 trials that are investigating the efficacy, safety, and pharmacokinetics/pharmacodynamics of intravitreal vamikibart in the treatment of UME secondary to NIU. The primary endpoint includes the proportion of patients with a ≥15 letter BCVA improvement from baseline to week 16 on treatment. Secondary endpoints include changes in BCVA and central subfield thickness (CST), rescue medication requirements, adverse events, and concentrations of IL-6 and vamikibart in aqueous humor and serum.24 The phase 3 trials were designed based on positive results from the phase 1 DOVETAIL study, wherein there was improvement in BCVA and CST in patients treated with vamikibart (3 dosing levels: 0.25 mg, 1 mg, and 2.5 mg) with a favorable safety profile.25
KSI-101 (Kodiak Sciences) is another intravitreal bispecific protein that targets IL-6 and vascular endothelial growth factor (VEGF) and is currently being tested in the phase 1b APEX trial for UME with plans for the phase 2/3 PEAK and PINNACLE studies.
SZN-8143 (Surrozen) is an investigational tri-specific antibody that is an antagonist of IL-6 and VEGF and an agonist of Frizzled-4 (FZD-4, which activates the Wnt signaling pathway involved in retinal vascular development). It currently is under preclinical investigation for retinal diseases but has not yet entered phase 1 clinical trials.
Conclusions
IL-6 has essential roles in inflammation and immune response. Targeting the IL-6 signaling pathway has therapeutic potential in the treatment of NIU. The monoclonal antibodies discussed in this review—several of which are FDA-approved for systemic immune-mediated diseases—show promise in managing NIU. These therapies are especially applicable to those patients who have refractory disease after management with antimetabolites and other biologics such as anti-TNF therapy. With future investigations, we will gain more insight into the long-term safety and efficacy of these therapies in treating patients with NIU. RP
References
1. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi:10.1101/cshperspect.a016295
2. Lin P. Targeting interleukin-6 for noninfectious uveitis. Clin Ophthalmol. 2015;9:1697-1702. doi:10.2147/OPTH.S68595
3. Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878-888. doi:10.1016/j.bbamcr.2011.01.034
4. Ma M, Jiang W, Zhou R. DAMPs and DAMP-sensing receptors in inflammation and diseases. Immunity. 2024;57(4):752-771. doi:10.1016/j.immuni.2024.03.002
5. Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249(1):158-175. doi:10.1111/j.1600-065X.2012.01146
6. Li D, Wu M. Pattern recognition receptors in health and diseases. Signal Transduct Target Ther. 2021;6(1):291. doi:10.1038/s41392-021-00687-0
7. Karkhur S, Hasanreisoglu M, Vigil E, et al. Interleukin-6 inhibition in the management of noninfectious uveitis and beyond. J Ophthalmic Inflamm Infect. 2019;9:17. doi:10.1186/s12348-019-0182-y
8. Reeh H, Rudolph N, Billing U, et al. Response to IL-6 trans- and IL-6 classic signalling is determined by the ratio of the IL-6 receptor α to gp130 expression: fusing experimental insights and dynamic modelling. Cell Commun Signal. 2019;17(1):46. doi:10.1186/s12964-019-0356-0
9. Wang J, Sun Q, Zhang J, Wang H, Liu H. Classical signaling and trans-signaling pathways stimulated by megalobrama amblycephala IL-6 and IL-6R. Int J Mol Sci. 2022;23(4):2019. doi:10.3390/ijms23042019
10. Schumertl T, Lokau J, Rose-John S, Garbers C. Function and proteolytic generation of the soluble interleukin-6 receptor in health and disease. Biochim Biophys Acta Mol Cell Res. 2022;1869(1):119143. doi:10.1016/j.bbamcr.2021.119143
11. Heinrich PC, Behrmann I, Müller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334:297-314. doi:10.1042/bj3340297
12. Hu X, Li J, Fu M, Zhao X, Wang W. The JAK/STAT signaling pathway: from bench to clinic. Signal Transduct Target Ther. 2021;6(1):1-33. doi:10.1038/s41392-021-007911
13. Dienz O, Rincon M. The effects of IL-6 on CD4 T cell responses. Clin Immunol. 2009;130(1):27-33. doi:10.1016/j.clim.2008.08.018
14. Okada M, Kitahara M, Kishimoto S, Matsuda T, Hirano T, Kishimoto T. IL-6/BSF-2 functions as a killer helper factor in the in vitro induction of cytotoxic T cells. J Immunol. 1988;141(5):1543-1549.
15. Sepah YJ, Sadiq MA, Chu DS, et al. Primary (month 6) outcomes of the STOP-Uveitis Study: evaluating the safety, tolerability, and efficacy of tocilizumab in patients with noninfectious uveitis. Am J Ophthalmol. 2017;183:71-80. doi:10.1016/j.ajo.2017.08.019
16. Atienza-Mateo B, Calvo-Río V, Beltrán E, et al. Anti-interleukin 6 receptor tocilizumab in refractory uveitis associated with Behçet’s disease: multicentre retrospective study. Rheumatol (Oxford). 2018;57(5):856-864. doi:10.1093/rheumatology/kex480
17. Ramanan AV, Dick AD, Guly C, et al. Tocilizumab in patients with anti-TNF refractory juvenile idiopathic arthritis-associated uveitis (APTITUDE): a multicentre, single-arm, phase 2 trial. Lancet Rheumatol. 2020;2(3):e135-e141. doi:10.1016/S2665-9913(20)30008-4
18. Calvo-Río V, Santos-Gómez M, Calvo I, et al. Anti-interleukin-6 receptor tocilizumab for severe juvenile idiopathic arthritis–associated uveitis refractory to anti–tumor necrosis factor therapy: a multicenter study of twenty-five patients. Arthritis Rheumatol. 2017;69(3):668-675. doi:10.1002/art.39940
19. Vegas-Revenga N, Calvo-Río V, Mesquida M, et al. Anti-IL6-receptor tocilizumab in refractory and noninfectious uveitic cystoid macular edema: multicenter study of 25 patients. Am J Ophthalmol. 2019;200:85-94. doi:10.1016/j.ajo.2018.12.019
20. Tappeiner C, Mesquida M, Adán A, et al. Evidence for tocilizumab as a treatment option in refractory uveitis associated with juvenile idiopathic arthritis. J Rheumatol. 2016;43(12):2183-2188. doi:10.3899/jrheum.160231
21. Heissigerová J, Callanan D, de Smet MD, et al. Efficacy and safety of sarilumab for the treatment of posterior segment noninfectious uveitis (SARIL-NIU): the phase 2 SATURN study. Ophthalmology. 2019;126(3):428-437. doi:10.1016/j.ophtha.2018.09.044
22. Grewal DS, Jaffe GJ, Keenan RT. Sarilumab for recalcitrant cystoid macular edema in non-paraneoplastic autoimmune retinopathy. Retin Cases Brief Rep. 2021;15(5):504-508. doi:10.1097/ICB.0000000000000872
23. Deaner JD, Jaffe GJ, Keenan RT, Carnago L, Grewal DS. Anti-interleukin-6 antibodies for autoimmune retinopathy with macular edema. Ophthalmol Retina. 2022;6(1):91-93. doi:10.1016/j.oret.2021.08.008
24. Sharma S, Suhler E, Lin P, et al. A novel intravitreal anti-IL-6 monoclonal antibody for uveitic macular edema (UME): preliminary results from the phase 1 DOVETAIL study. Invest Ophthalmol Vis Sci. 2023;64(8):5100.
25. Suhler EB, Steeples L, Elze M, et al. IL-6 inhibition with vamikibart in patients with uveitic macular edema: phase 3 MEERKAT and SANDCAT trials. Invest Ophthalmol Vis Sci. 2024;65(7):2609.
26. Hassan M, Sadiq MA, Ormaechea MS, et al. Utilisation of composite endpoint outcome to assess efficacy of tocilizumab for non-infectious uveitis in the STOP-Uveitis Study. Br J Ophthalmol. 2023;107(8):1197-1201. doi:10.1136/bjophthalmol-2021-320604
27. Leclercq M, Andrillon A, Maalouf G, et al. Anti-tumor necrosis factor α versus tocilizumab in the treatment of refractory uveitic macular edema: a multicenter study from the French Uveitis Network. Ophthalmology. 2022;129(5):520-529. doi:10.1016/j.ophtha.2021.11.013
28. Leclercq M, Jacquot R, Charbonnier C, et al. Comparative effectiveness of intravenous versus subcutaneous tocilizumab for refractory uveitis: a retrospective analysis. Ophthalmology. Published online May 19, 2025. doi:10.1016/j.ophtha.2025.05.014