A novel treatment for intermediate dry age-related macular degeneration (AMD), photobiomodulation (PBM) of the retina, has demonstrated good results in clinical trials.1,2 In November 2024, the Valeda Light Delivery System (Alcon) received FDA authorization.3,4 Physicians are excited to finally offer a treatment plan other than vitamins, dietary changes, and weight loss.5-7 In contrast to intravitreal injections of complement inhibitors, which can slow but not reverse the progression of geographic atrophy in advanced dry AMD, PBM has shown potential to improve visual function in some patients.8,9
Photobiomodulation does not require anesthesia or any other medication such as dilating agents. Consequently, it is not performed in an ambulatory surgery center or operating room. It is usually performed in a clinic setting, such as a doctor’s office (Figure 1). It may also be performed in a dedicated suite under physician supervision. Medicare does not cover claims for reimbursement of a hospital facility fee for PBM.
Who Administers PBM?
State laws and regulations define the roles and responsibilities of health care workers, and their interpretation is carried out by the appropriate state boards. The Medical Board oversees physicians, surgeons, and medical assistants, while the Physician Assistant Board regulates physician assistants (PAs). The Board of Optometry is responsible for optometrists, and the Board of Nursing oversees registered nurses, licensed vocational nurses, nurse practitioners (NPs), and other advanced-practice nurses.

Figure 1. Treatment for intermediate dry age-related macular degeneration with the Valeda Light Delivery System (Alcon) is typically delivered in a clinical setting. Patients sit at the device, while the photobiomodulation therapy is delivered directly to the retina.
Nationwide, there are differences in laws and their interpretation; readers should check their state board for specific information. During the research for this article, limitations on scope of practice for optometrists, physician assistants, nurse practitioners, and medical assistants were identified. Some examples follow.
In California under the Business and Professional Code §3401(a)(5)(F)(xii), optometrists are permitted “Use of noninvasive devices delivering intense pulsed light therapy or low-level light therapy that do not rely on laser technology, limited to treatment of conditions of the adnexa.” Significantly, PBM is applied to the retina and not the adnexa.
The California Physician Assistant Practice Act provides that PAs may perform procedures if all of the following conditions are met:
- The PA is supervised by a physician;
- Procedures are defined within a PA practice agreement;
- The PA is trained on the procedure; has competency; and
- The procedures are ordered by a physician and are patient specific.
The Act does not authorize PAs to perform refractions, prescribe glasses or contact lenses, or do routine visual screening.
Article 8 of the California Business and Professional Code pertains to NPs. It requires the following:
- The NP is a licensed registered nurse with a graduate degree in nursing;
- The NP has completed an NP training program approved by the Board of Nursing;
- The NP adheres to Board of Nursing standards;
- The NP has established clinical competency;
- The NP follows standardized procedures; and
- The physician be available by phone (physical presence of the physician is not required).
Within CPT, PAs and NPs are referred to, along with some other health care professionals, as “qualified health care professionals” (QHPs). The characterization is important to identify providers who can be credentialed with health care plans and submit claims for reimbursement.
The California Medical Board has carefully described the limitations of medical assistants (MA). They state, “Medical assistants are unlicensed, and may only perform basic administrative, clerical and technical supportive services as permitted by law. An unlicensed person may not diagnose or treat or perform any task that is invasive or requires assessment. The responsibility for the appropriate use of unlicensed persons in health care delivery rests with the physician.”10 Table 1 summarizes what medical assistants can and cannot do.
Most states share this point of view, which means that an MA cannot perform PBM even if directly supervised by a physician. Research on this point found considerable disagreement about what MAs may do, so the reader is encouraged to carefully investigate the matter and not blithely proceed. Significantly, payors are oriented to reimburse services personally performed by a physician or QHP while services performed by an MA under supervision of a physician are not equivalent. During discussions with all the Medicare Administrative Contractors (MACs) in March 2025, the medical directors said that PBM should not be administered by MAs or ophthalmic technicians, even if supervised by a physician.
Coding and Billing for PBM
On January 1, 2025, Category III CPT code 0936T (“photobiomodulation of retina, single session”) was inaugurated. The term “session” refers to a treatment on a single day, with 1 unit reported per claim. CPT instructs, “For bilateral procedure, report 0936T with modifier 50.” This code reports a procedure that was personally performed by an ophthalmologist, optometrist, or QHP in compliance with state laws and regulations.
In 2019, CPT code 0552T (“low-level laser therapy, dynamic photonic and dynamic thermokinetic energies, provided by a physician or other QHP”) was added to the CPT manual. The term “laser” does not apply to PBM of the retina, which uses low-intensity, noncoherent, multiwavelengths of light, so this code cannot be used to report it. HCPCS code S8948 (“application of a modality requiring constant provider attendance to one or more areas; low-level laser; each 15 minutes”) has the same difficulty and likewise cannot be used. In 2024, 97037 (“application of a modality to one or more areas; low-level laser therapy i.e., nonthermal and nonablative for postoperative pain reduction”) was added to the CPT manual. The phrase “postoperative pain reduction” as well as the term “laser” do not apply to PBM, so this code cannot be used on a claim. It is noteworthy that one MAC, National Government Services, published a policy that defines all of these codes as not reasonable and necessary and not payable by Medicare.11,12 Importantly, recent experience with 0936T in 2025 has been favorable and many claims have been paid.
Because PBM is considered a minor procedure, the modifier -25 rules for same-day examinations apply. In most cases, an eye exam is not billed on the same day as 0936T. An exception exists when the exam is performed for a separate and distinct reason unrelated to the minor procedure. For example, a patient may return for re-evaluation of floaters and a posterior vitreous detachment (PVD). During the examination, several drusen indicative of intermediate dry AMD and reduced best-corrected visual acuity (BCVA) of 20/40 OU may be noted. With the patient’s consent, PBM is performed OU. In this scenario, a claim for the eye exam with modifier -25 is supported by the floaters and PVD, not the intermediate dry AMD.
In the situation where PBM is performed by a QHP, CMS’s regulation on “incident to” services is germane.14 When a service satisfies the conditions for “incident to” billing, it is reimbursed based on 100% of the allowed amount for the procedure in the Medicare physician fee schedule. Those conditions require all the following:
- The QHP is credentialed with CMS;
- The QHP is employed by the entity billing for services;
- The procedure is ordered by a physician;
- There is direct supervision by a physician on the premises; ordering and supervising physician may be different;
- Services are provided in a noninstitutional setting;
- The service is for an established patient of the practice, not a new patient; and
- The claim is filed with the PIN of the supervising physician.
If the “incident to” requirements are not satisfied, the reimbursement is reduced to 85% of the allowed amount.
Chart Documentation
Before a physician orders PBM, an eye exam, usually with dilation, is necessary to establish medical necessity for the procedure. At the same time, determination of BCVA is made. In addition, ancillary diagnostic tests are usually performed that might include: fundus photography, fundus autofluorescence, optical coherence tomography (OCT), or OCT angiography.
During the process of getting the patient’s informed consent to proceed with the therapy, the physician and/or office staff must explain that coverage of PBM by health insurance plans is uncertain and the patient might be financially responsible for the procedure. This is accomplished with a financial waiver. For Medicare Part B, an Advance Beneficiary Notice of Noncoverage (ABN) is used; for Medicare Part C, a determination of benefits is made; for other payors, a Notice of Exclusion from Health Plan Benefits is used.
A minor procedure such as photobiomodulation (PBM) should be documented in the medical record with a detailed report that includes the date of service, patient name, diagnosis, proceduralist’s name, title of the procedure, indication for the procedure, description of the procedure, discharge instructions, and a signature that meets payor guidelines.
Payor Coverage
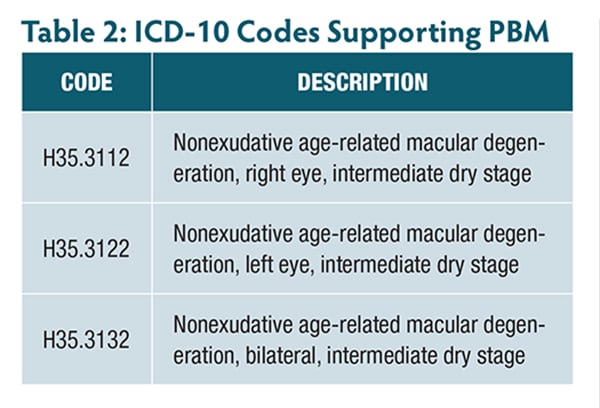
At present, payors have not published a local coverage policy that formally declares where, when, why, and how often they will pay for PBM, so providers must file claims to obtain a determination. The directions for use for the Valeda Light Delivery System15 provide the needed information to establish which ICD-10 codes would likely support payment for PBM (Table 2).
When counseling patients about treatment with PBM and their potential financial responsibility for noncovered services, clinicians can note that off-label use is generally not covered by insurance, and conversely, coverage is more likely for approved indications. The Valeda Light Delivery System is intended to provide improved visual acuity in patients with BCVA of 20/32 through 20/70 and who have dry age-related macular degeneration (AMD) characterized by the presence of at least 3 medium drusen (>63 μm and ≤125 μm in diameter), large drusen (>125 μm in diameter), or noncentral geographic atrophy, AND the absence of neovascular maculopathy or center-involving geographic atrophy.15
The following frequently asked questions illustrate common scenarios and help clarify when the use of PBM with the Valeda system is considered on-label versus off-label.
Q: If PBM is used to treat an eye with both wet and dry AMD, is this on-label or off-label use?
A: This would be off-label use, because this is not part of the indications for use of the Valeda Light Delivery System.
Q: If PBM is used to treat a patient with wet AMD in one eye and intermediate dry AMD in the opposite eye, is this on-label or off-label use?
A: This would be on-label use for the eye with intermediate dry AMD, because that is part of the indications for use of the Valeda Light Delivery System. It would be off-label use for the eye with wet AMD, because that is not part of the indications for use of the Valeda Light Delivery System.
Q: If PBM is used to treat an eye with intermediate dry AMD as well as center-involving geographic atrophy, is this on-label or off-label use?
A: This would be off-label use, because this is not part of the indications for use of the Valeda Light Delivery System.
Q: If PBM is used to treat an eye with early dry AMD and 20/20 BCVA, is this on-label or off-label use?
A: This would be off-label use, because 20/20 vision is not part of the indications for use of the Valeda system.
Payment for Photobiomodulation
Within the 2025 Medicare Physician Fee Schedule, CMS did not assign an allowed amount to Category III CPT code 0936T. That is not oversight but the expected action because MACs typically make a determination on a claim-by-claim basis for temporary codes. Clients report payments for claims that vary geographically and between payors.
Conclusion
Photobiomodulation provides physicians with a means to effectively treat intermediate dry AMD; that’s exciting. Until now, there wasn’t much to recommend other than vitamins, dietary changes, and weight loss. With this diagnosis, patients were frightened by the threat of vision loss, so they are understandably enthused by a therapy that may mitigate the problem to some degree. Some essential practice management requirements for providing this service are already in place: FDA authorization, a CPT code, some early paid claims, and a mechanism for patient payment of noncovered claims. In the United States, there are an estimated 18.3 million patients with dry AMD,16 so PBM represents a major advance for eye care. RP
References
1. Valter K, Tedford SE, Eells JT, Tedford CE. Photobiomodulation use in ophthalmology—an overview of translational research from bench to bedside. Front Ophthalmol (Lausanne). 2024;4:1388602. doi:10.3389/fopht.2024.1388602
2. Munk MR, Valter K. Photobiomodulation as an innovative and promising treatment for retinal disease. Retinal Physician. 2022;19(3)36-39. https://retinalphysician.com/issues/2022/april/photobiomodulation-as-an-innovative-and-promising-treatment-for-retinal-disease/
3. US Food and Drug Administration. DEN230083, letter to LumiThera, Inc. November 4, 2024. Accessed October 23, 2025. https://www.accessdata.fda.gov/cdrh_docs/pdf23/DEN230083.pdf.
4. Mukamal R. FDA authorizes light therapy for dry AMD. American Academy of Ophthalmology. January 21, 2025. Accessed October 16, 2025. https://www.aao.org/eye-health/news/light-therapy-photobiomodulation-dry-amd-ga
5. Brzostowicki D. New therapy brings hope for dry AMD vision loss. MD Edge. January 9, 2025. Accessed October 23, 2025. https://www.mdedge.com/familymedicine/article/272135/preventive-care/new-therapy-brings-hope-dry-amd-vision-loss
6. Rosen R, Photobiomodulation: innovation on the horizon for dry AMD. Retina Today. May/June 2024. Accessed October 23, 2025. https://retinatoday.com/articles/2024-may-june/photobiomodulation-innovation-on-the-horizon-for-dry-amd
7. Chichan H, Aldujaly IH, Michalakis K, Kanal L. Photobiomodulation in ocular therapy: current status and future perspectives. Int J Ophthalmol. 2025;18(2):351-357. doi:10.18240/ijo.2025.02.20
8. Boyer DS, Asanad S. Photobiomodulation therapy delays dry AMD progression. Retinal Physician. March 1, 2025. Accessed October 23, 2025. https://www.retinalphysician.com/issues/2025/march/photobiomodulation-therapy-delays-dry-amd-progression/
9. Kaymak H, Schwahn H. Photobiomodulation as a treatment of dry AMD. Retina Today. May/June 2020. Accessed October 23, 2025. https://retinatoday.com/articles/2020-may-june/photobiomodulation-as-a-treatment-in-dry-amd
10. California Medical Board. Is your medical assistant practicing beyond their scope of training? Accessed October 23, 2025. https://www.mbc.ca.gov/Licensing/Physicians-and-Surgeons/Practice-Information/Medical-Assistants.aspx
11. National Government Services. LCD L33631. December 18, 2019. Accessed October 23, 2025. https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdId=33631&ver=51
12. National Government Services. LCA A56566. December 19, 2024. Accessed October 23, 2025. https://www.cms.gov/medicare-coverage-database/view/article.aspx?articleid=56566
13. CMS. Medicare Claims Processing Manual Chapter 12, §40.2(A)(8). December 19, 2024. Accessed October 23, 2025. https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/clm104c12.pdf
14. CMS. Medicare Benefit Policy Manual Chapter 15, §60. April 11, 2025. Accessed October 23, 2025. https://www.cms.gov/regulations-and-guidance/guidance/manuals/downloads/bp102c15.pdf
15. Valeda Light Delivery System user manual. Accessed October 23, 2025. https://www.manualslib.com/manual/1831158/Valeda-Light-Delivery-System.html#manual
16. Prevent Blindness. Prevalence of age-related macular degeneration. Accessed October 23, 2025. https://preventblindness.org/amd-prevalence-vehss