The DRCR Retina Network (formerly known as the Diabetic Retinopathy Clinical Research Network) was founded in 2002 with the goal of conducting high-quality, large clinical trials focused on diabetic retinopathy (DR) and diabetic macular edema (DME), 2 common causes of vision loss in patients with diabetes. The National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases provide funding, with additional support coming from nonprofit organizations and industry partnerships. The network’s inaugural chair was Lloyd Paul Aiello, MD, PhD, who was succeeded by Neil M. Bressler, MD, Lee Jampol, MD, and then by the current co-chairs Jennifer Sun, MD, MPH, and Daniel F. Martin, MD. Today, the DRCR Retina Network has grown to include more than 1,500 members, including investigators, coordinators, technicians, and coordinating center staff, and its collaborative efforts have produced many pivotal studies that have had an immense influence on the practice patterns of retina specialists around the world.
Pivotal DRCR Retina Network Studies
One of the DRCR Retina Network’s most significant early contributions to the field was the demonstration that intravitreal anti–vascular endothelial growth factor (anti-VEGF) injections were highly effective for the treatment of DME. Historically, macular laser had been the standard of care for the treatment of DME. The observation that VEGF levels were elevated in eyes with DR raised the possibility that inhibiting this pathway may be beneficial.1 Protocol I showed that intravitreal ranibizumab (Lucentis; Genentech) in combination with prompt or deferred laser treatment resulted in superior visual acuity (VA) outcomes in patients with DME compared to laser treatment with sham injections.2 Protocol I revolutionized the field by establishing a new treatment paradigm for DME.
Subsequent DRCR Retina Network clinical trials provided additional data to guide treatment of DME. Protocol T compared the efficacy of ranibizumab, bevacizumab (Avastin; Genentech), and aflibercept (Eylea; Regeneron) for patients with center-involved DME (CI-DME). All 3 anti-VEGF agents resulted in similar VA outcomes at 1 year for patients with mild vision loss (20/32 to 20/40), but aflibercept resulted in superior VA outcomes for patients with moderate or worse vision loss (20/50 or worse).3 Protocol V focused on treatment options for eyes with CI-DME and good VA (20/25 or better). This study found that there was no significant difference in vision loss at 2 years for patients initially treated with aflibercept, laser, or observation alone, demonstrating that treatment of DME can be deferred in patients with good VA.4 More recently, Protocol AC compared treatment of CI-DME with aflibercept monotherapy to treatment with bevacizumab first and a switch to aflibercept if the response was suboptimal. There was no difference in visual outcomes at 2 years, suggesting that it is acceptable to start patients on bevacizumab for initial treatment of DME.5
Early DRCR Retina Network studies focusing on DME also suggested that anti-VEGF treatment had beneficial effects on DR severity. For instance, patients receiving ranibizumab in Protocol I were less likely to show worsening of DR, had lower incidence of vitreous hemorrhage, and were less likely to require panretinal photocoagulation (PRP).2 At the time, PRP was the only established treatment for proliferative diabetic retinopathy (PDR), with the Diabetic Research Study (DRS) demonstrating that PRP could reduce the incidence of severe vision loss by 50%.6
Protocol S investigated whether intravitreal anti-VEGF treatment was a viable treatment strategy for PDR. Patients with PDR with or without DME were randomized to receive ranibizumab or PRP, with supplemental ranibizumab given as needed for DME in both groups. Visual acuity outcomes for patients in the ranibizumab group were noninferior to those undergoing PRP at 2 years, and VA outcomes remained similar at 5 years.7,8 Patients in the ranibizumab group showed less peripheral visual field loss (yet at 5 years the difference was not as significant) and were less likely to develop vision-impairing DME.
Protocol W extended these findings to severe nonproliferative diabetic retinopathy (NPDR). In this study, patients with moderately severe to severe NPDR (Diabetic Retinopathy Severity Scale score [DRSS] 43-53) were randomized to protocol-specified aflibercept treatment or observation with aflibercept given only if they developed PDR or vision-impairing CI-DME. At both 2 and 4 years, patients in the aflibercept group were less likely to have developed PDR or vision-impairing CI-DME. However, preventive treatment did not lead to a VA benefit at either timepoint.9,10
Taken together, these studies demonstrate an important role for anti-VEGF treatment in the management of DME and DR. In the case of PDR, PRP will continue to have an important role in the real-world setting where patient follow-up may be less reliable.
Expanded Scope of the DRCR Retina Network
In 2018, the DRCR Retina Network expanded its scope to focus on all retinal diseases, applying the same collaborative research strategy that had already proven so successful in the study of diabetic eye disease. Protocol AG and AH studied pneumatic vitreolysis for eyes with vitreomacular traction with or without full-thickness macular holes (FTMH). Pneumatic vitreolysis induced hyaloid release in most eyes and resulted in macular hole closure in about one-third of eyes, but the studies were terminated early due to higher than expected rates of retinal tears and retinal detachments.11 Protocol AJ is aiming to identify proteomic abnormalities in eyes with FTMH by comparing vitreous samples obtained during surgical repair to samples from eyes undergoing surgery for vitreous opacities; analysis of this data is ongoing. Protocol AK was a pilot study examining the feasibility of monitoring retinal fluid in neovascular age-related macular degeneration (nAMD) with a home optical coherence tomography (OCT) device.12 The results showed that at-home scanning could effectively assess retinal fluid and laid the groundwork for Protocol AO, which is currently enrolling.
Protocol AF
Although intravitreal anti-VEGF injections and PRP effectively treat DR, the availability of an oral agent that could slow retinopathy progression might significantly decrease treatment burden. Previous studies have suggested that fenofibrate, a peroxisome proliferator-activated receptor alpha agonist used to treat hypercholesterolemia and hyperlipidemia, may have beneficial effects on DR.13,14 In Protocol AF, patients with mild and moderate NPDR (DRSS 35-47) without CI-DME are randomized to receive fenofibrate or placebo, and are subsequently followed for up to 6 years. The primary objective is to determine whether fenofibrate prevents worsening of DR. A substudy with Protocol AF participants will examine the relationship between glycemic parameters and progression of DR using continuous glucose monitoring. Another ancillary study aims to identify metabolic and genomic biomarkers that may predict DR progression. These studies have the potential to provide novel insights into the mechanism of DR progression and how it might be prevented.
Protocol AL
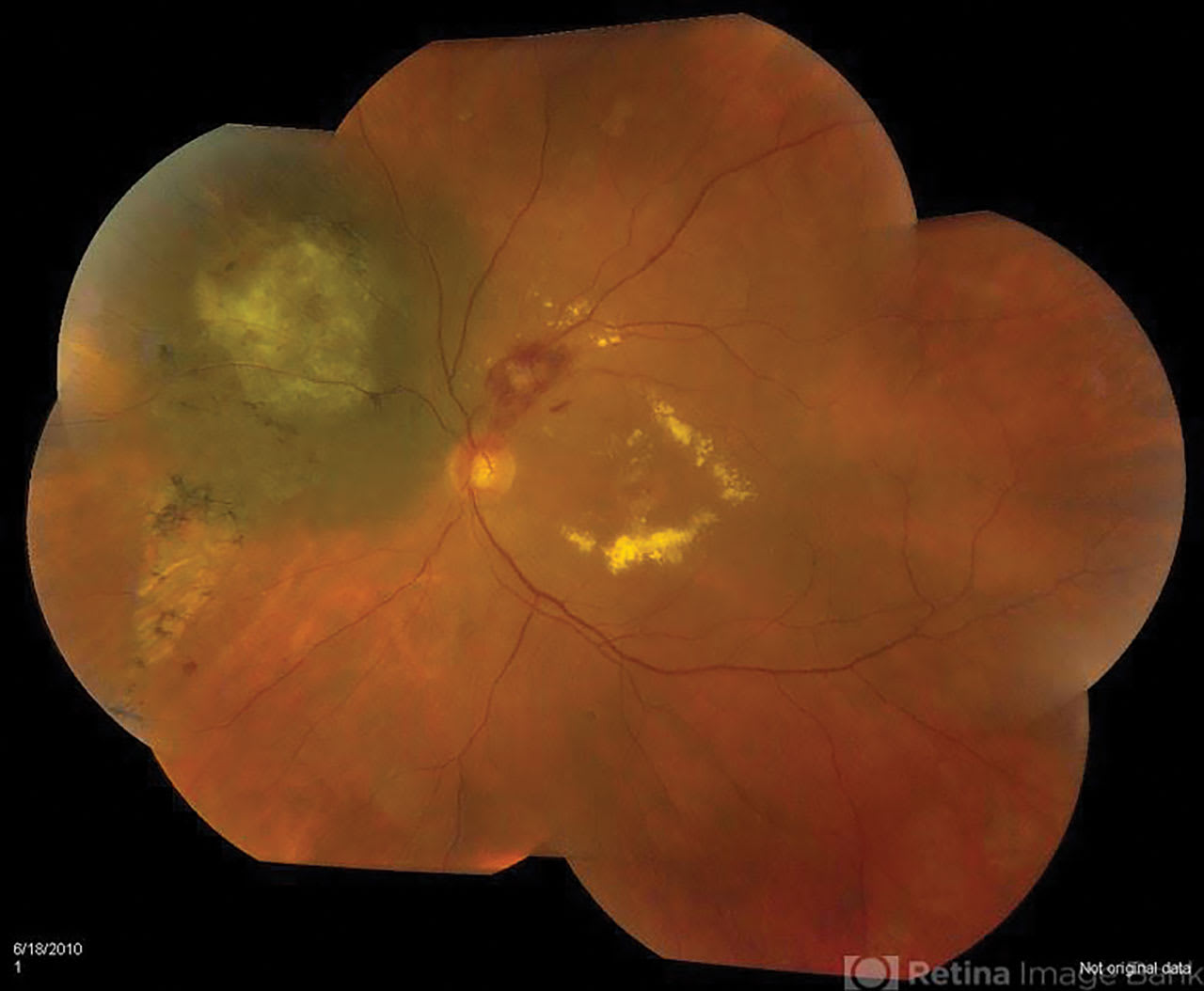
Figure 1. Radiation retinopathy with macular exudates following radioactive plaque therapy for choroidal melanoma. This image was originally published in the Retina Image Bank. Jason S. Calhoun. Melanoma With Radiation Retinopathy. Retina Image Bank. 2013; 7797. Courtesy and copyright of the American Society of Retina Specialists.
Radiation retinopathy is a common complication of I-125 plaque therapy for choroidal melanoma (Figure 1). It is characterized by capillary leakage, macular edema, and retinal ischemia, potentially leading to severe vision loss. In the Collaborative Ocular Melanoma Study (COMS), 43% of subjects with medium-sized choroidal melanoma suffered a VA decline to 20/200 or worse 3 years following plaque radiotherapy.15 Intravitreal anti-VEGF and corticosteroid treatments have been used to mitigate macular edema associated with radiation retinopathy, but no large randomized controlled trials have examined the best treatment approach. Protocol AL was designed to help define the optimal strategy for prevention of vision loss associated with radiation retinopathy. Patients undergoing radioactive plaque placement with a planned dose of at least 30 Gy to the macula are randomized to faricimab (Vabysmo; Genentech) administered every 3 months, fluocinolone acetonide 0.19 mg implant (Iluvien; ANI Pharmaceuticals) administered at randomization and 24 months, or observation alone. The primary outcomes are the change in VA at 3 years and the rate of severe vision loss at 3 years, with presence of macular edema on OCT serving as a key secondary outcome.
Protocol AM
Epiretinal membranes are among the most common vitreoretinal problems requiring surgery, but the optimal timing of treatment remains unclear. Protocol AM is a 3-year study enrolling patients with symptomatic ERMs and VA of 20/40 or better. Patients are randomized to immediate surgery or deferred surgery, whereby surgery would only be performed if protocol-defined criteria are met. The primary outcome is VA at 3 years, with secondary outcomes focusing on other measures of visual function such as metamorphopsia and reading speed.
Protocol AO

Figure 2. The US Food and Drug Administration recently cleared the Scanly home OCT device for use in patients with nAMD, and it is expected to become commercially available soon. Image courtesy Notal Vision.
The FDA recently granted de novo marketing authorization for the first commercial home OCT device (Scanly; Notal Vision) (Figure 2), which has the potential to facilitate management of nAMD. However, there have not been any large randomized controlled trials comparing outcomes of home OCT-guided treatment to standard of care treatment. Moreover, questions remain about how macular fluid fluctuates over time and what the optimal thresholds are for triggering patients to return to clinic.
Protocol AO is a 2-year study enrolling treatment-naïve patients with newly diagnosed nAMD. Patients are randomized to a home OCT-guided treatment algorithm or a treat-and-extend algorithm designed to mirror a commonly used approach to managing patients with nAMD. All patients are treated with faricimab. The primary outcomes are VA and number of injections at 2 years. Home OCT has the potential to reduce treatment burden by enabling more personalized care for nAMD, and Protocol AO will provide important insights into how this new technology can be best utilized.
Protocol AP
Proliferative diabetic retinopathy is a serious, vision-threatening complication of diabetes. PRP and anti-VEGF therapy are viable treatments for PDR, but both approaches have limitations. Many eyes that undergo PRP still require additional treatment for persistent PDR activity. PRP can also lead to visual field loss and worsening of DME. Anti-VEGF monotherapy is highly effective, but treatment noncompliance can lead to recurrent PDR activity and associated sequelae such as vitreous hemorrhage and tractional retinal detachment (TRD). In contrast to medical management, vitrectomy surgery is a compelling treatment approach for PDR because the posterior hyaloid is speculated to contribute to PDR worsening and TRD development.
Protocol AP compares medical management with PRP and anti-VEGF treatment to surgical management with vitrectomy and intraoperative PRP. The primary outcomes are VA and number of in-office treatments needed at 3 years. Key secondary outcomes are the development of complications such as vitreous hemorrhage and TRD. Developing a standardized surgical approach to PDR and comparing outcomes to medical management has the potential to significantly alter management of this condition.
Conclusion
For more than 2 decades, DRCR Retina Network clinical trials have provided an important source of high-quality data to guide treatment of retinal disease. Today, the network continues to be driven by its original mission of studying retinal complications of diabetes while also expanding its scope to other important questions in the field of medical and surgical retina (Table 1). Through continued collaboration and the recruitment of additional research sites, the DRCR Retina Network seeks to further advance the treatment of vision-threatening retinal diseases. RP
Email drcrnet@jaeb.org if you are interested in learning more about joining the Network!
References
1. Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480-1487. doi:10.1056/NEJM199412013312203
2. Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064-1077.e35. doi:10.1016/j.ophtha.2010.02.031
3. Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372(13):1193-1203. doi:10.1056/NEJMoa1414264
4. Baker CW, Glassman AR, Beaulieu WT, et al. Effect of initial management with aflibercept vs laser photocoagulation vs observation on vision loss among patients with diabetic macular edema involving the center of the macula and good visual acuity: a randomized clinical trial. JAMA. 2019;321(19):1880-1894. doi:10.1001/jama.2019.5790
5. Jhaveri CD, Glassman AR, Ferris FL, et al. Aflibercept monotherapy or bevacizumab first for diabetic macular edema. N Engl J Med. 2022;387(8):692-703. doi:10.1056/NEJMoa2204225
6. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. The Diabetic Retinopathy Study Research Group. Ophthalmology. 1981;88(7):583-600.
7. Writing Committee for the Diabetic Retinopathy Clinical Research Network, Gross JG, Glassman AR, et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2015;314(20):2137-2146. doi:10.1001/jama.2015.15217
8. Gross JG, Glassman AR, Liu D, et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136(10):1138-1148. doi:10.1001/jamaophthalmol.2018.3255
9. Maturi RK, Glassman AR, Josic K, et al. Effect of intravitreous anti–vascular endothelial growth factor vs sham treatment for prevention of tision-threatening complications of diabetic retinopathy: the Protocol W randomized clinical trial. JAMA Ophthalmol. 2021;139(7):701-712. doi:10.1001/jamaophthalmol.2021.0606
10. Maturi RK, Glassman AR, Josic K, et al. Four-year visual outcomes in the Protocol W randomized trial of intravitreous aflibercept for prevention of vision-threatening complications of diabetic retinopathy. JAMA. 2023;329(5):376-385. doi:10.1001/jama.2022.25029
11. Chan CK, Mein CE, Glassman AR, et al. Pneumatic vitreolysis with perfluoropropane for vitreomacular traction with and without macular hole: DRCR Retina Network Protocols AG and AH. Ophthalmology. 2021;128(11):1592-1603. doi:10.1016/j.ophtha.2021.05.005
12. Blinder KJ, Calhoun C, Maguire MG, et al. Home OCT imaging for newly diagnosed neovascular age-related macular degeneration: a feasibility study. Ophthalmol Retina. 2024;8(4):376-387. doi:10.1016/j.oret.2023.10.012
13. Elam M, Lovato L, Ginsberg H. The ACCORD-Lipid study: implications for treatment of dyslipidemia in Type 2 diabetes mellitus. Clin Lipidol. 2011;6(1):9-20. doi:10.2217/clp.10.84
14. Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849-1861. doi:10.1016/S0140-6736(05)67667-2
15. Melia BM, Abramson DH, Albert DM, et al. Collaborative ocular melanoma study (COMS) randomized trial of I-125 brachytherapy for medium choroidal melanoma. I. Visual acuity after 3 years COMS report no. 16. Ophthalmology. 2001;108(2):348-366. doi:10.1016/s0161-6420(00)00526-1