A post hoc imaging analysis from the phase 1 HELIOS trial suggests that a single intravitreal injection of OTX-TKI (Ocular Therapeutix) may reduce retinal leakage for up to 12 months in patients with diabetic retinopathy without diabetic macular edema. Results from the analysis, which was based on changes measured over time using ultra-widefield fluorescein angiography, were presented at the 2025 American Society of Retina Specialists (ASRS) meeting in Long Beach, California.
“Leakage is an important metric or a sign of disease activity in diabetic retinopathy,” explained Katherine E. Talcott, MD, an associate professor of ophthalmology at Cleveland Clinic Lerner College of Medicine, Cole Eye Institute, who presented the data. “This post hoc analysis was performed to look at quantitative leakage on ultrawidefield fluorescence angiography images to be able to detect any differences.”
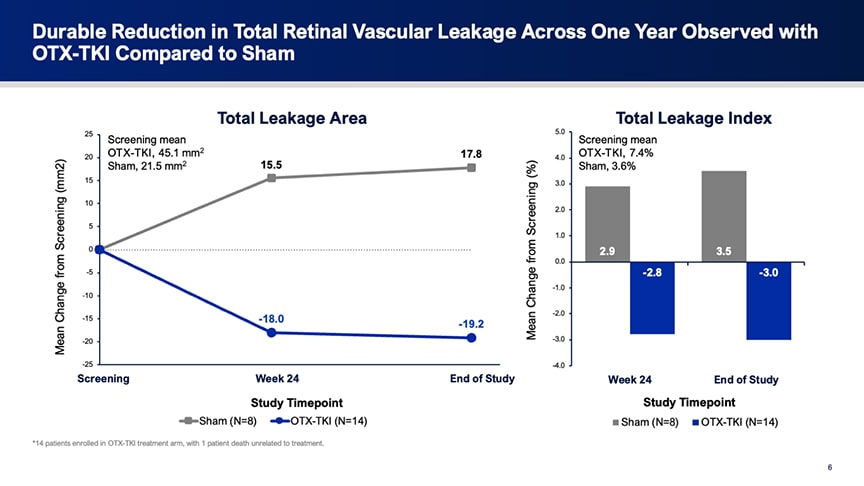
Figure 1. Quantitative analysis of retinal leakage in the HELIOS trial. In the sham group, leakage area and leakage index increased over the course of the study, consistent with progressive disease activity. In contrast, the OTX-TKI group showed a general reduction in leakage area and index, supporting a potential treatment effect.
OTX-TKI is a bioresorbable intravitreal implant composed of a polymer matrix combined with axitinib, a multitargeted tyrosine kinase inhibitor with a high affinity for VEGFR2. The implant is injected as a single rod into the vitreous cavity and gradually dissolves over 6 to 12 months, releasing the drug over time. The phase 1 HELIOS trial randomized patients 2:1 to receive either OTX-TKI or a sham injection at baseline. Participants had moderately severe to severe nonproliferative diabetic retinopathy, measured from level 47 to 53 on the Diabetic Retinopathy Severity Scale (DRSS), and were followed for a year.
While the primary endpoint of HELIOS was safety, investigators also evaluated changes in DRSS, visual acuity, central subfield thickness, and vision-threatening complications, said Dr. Talcott. The post hoc analysis focused on quantifying retinal leakage, using a machine learning–augmented imaging platform that calculated a leakage index—the percentage of the retina affected by hyperfluorescence—by segmenting ultrawidefield images and validating the output with trained human readers.
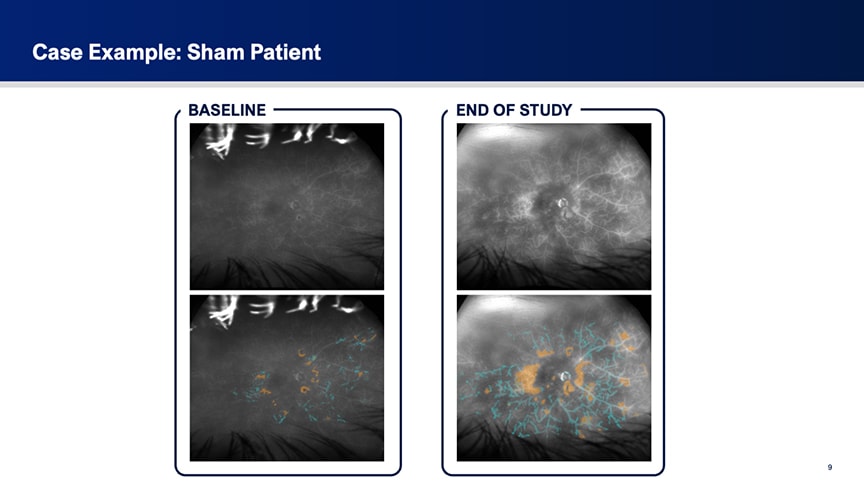
Figure 2. Ultrawidefield fluorescein angiography of a sham-treated patient in the HELIOS trial. Baseline leakage, indicated in teal (perivascular) and gold (general), markedly increased by the end of the study. Red areas represent overlapping leakage signals.
“Overall, you can see in the sham-treated group there was an increase in total leakage area as well as in total leakage index,” said Dr. Talcott. “This is in contrast with patients treated with OTX-TKI, who had a decrease in the leakage area (Figure 1). We saw that these results also hold up when we calculated the total leakage index. These results were the same when we looked at peripheral leakage area, as well as when we looked at posterior pole leakage area and index.”
To illustrate the findings, Dr. Talcott showed fluorescein angiography images from representative cases. One sham-treated patient showed progressive worsening of leakage over 12 months, with a marked increase in hyperfluorescent areas (Figure 2). In contrast, a patient in the OTX-TKI arm demonstrated substantial improvement over the same period (Figure 3).
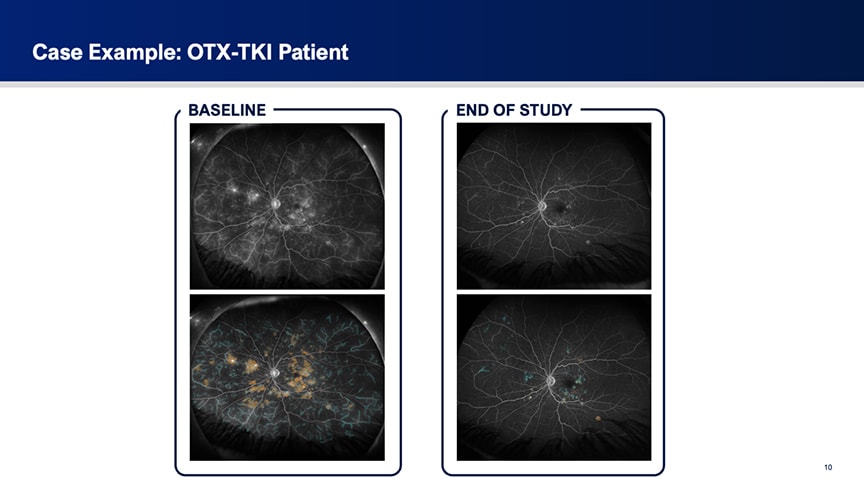
Figure 3. Ultrawidefield fluorescein angiography of an OTX-TKI–treated patient shows that significant baseline leakage decreased substantially by the end of the study.
Although Dr. Talcott cautioned that the analysis was limited by its small size, she concluded, “The post hoc analysis showed that a single OTX-TKI injection may provide sustained decrease in retinal leakage across the peripheral retina, posterior pole, and the total retinal area for up to 12 months. Taken with the other results from the phase 1 HELIOS study showing DRSS improvement as well as the absence of vision-threatening complications at week 48, this may support the use of this agent as a long-acting treatment option for diabetic retinopathy.”
Currently, OTX-TKI is also being studied in a pair of pivotal phase 3 trials for neovascular age-related macular degeneration, SOL-1 and SOL-R. RP








