The port delivery system with ranibizumab (Susvimo; Genentech) has demonstrated sustained vision outcomes over a 5-year period in patients with neovascular age-related macular degeneration (nAMD), according to data from the ongoing phase 3 Portal extension study presented at the 2025 American Society of Retina Specialists (ASRS) annual meeting in Long Beach, California.
John Kitchens, MD, of Retina Associates of Kentucky, reported data from the Portal study, which includes 352 participants originally enrolled in Archway, a randomized trial comparing Susvimo to monthly intravitreal injections of ranibizumab. Participants in Archway’s Susvimo arm (n=220) continued receiving biannual refills of the implanted port delivery system (PDS) in Portal. Those who had previously received monthly anti-VEGF intravitreal injections in Archway (n=132) transitioned to the PDS implant upon entering Portal and began receiving refills every 6 months.

Figure 1. Over 5 years, the port delivery system with ranibizumab (PDS) maintained disease control with about half of patients retaining 20/40 vision or better.
Over the 5-year period, Susvimo showed sustained efficacy in preserving vision and controlling disease activity. In the Susvimo cohort, mean best-corrected visual acuity (BCVA) declined from 74.4 letters at baseline to 67.6 letters at year 5. In the IVT-to-Susvimo cohort, BCVA declined from 76.3 to 68.6 letters over the same period. Approximately half of all participants had vision equivalent to 20/40 or better at year 5 (Figure 1).
“These patients had a maintenance of vision over those 5 years, essentially losing on average about 7 letters of visual acuity,” Dr. Kitchens reported. He noted that this BCVA decline in Portal compares favorably with outcomes of anti-VEGF treatment for nAMD in other real-world settings, noting that typical long-term losses in CATT, HORIZON, and SEVEN-UP ranged from 12 to 17 letters over similar intervals (Figure 2).
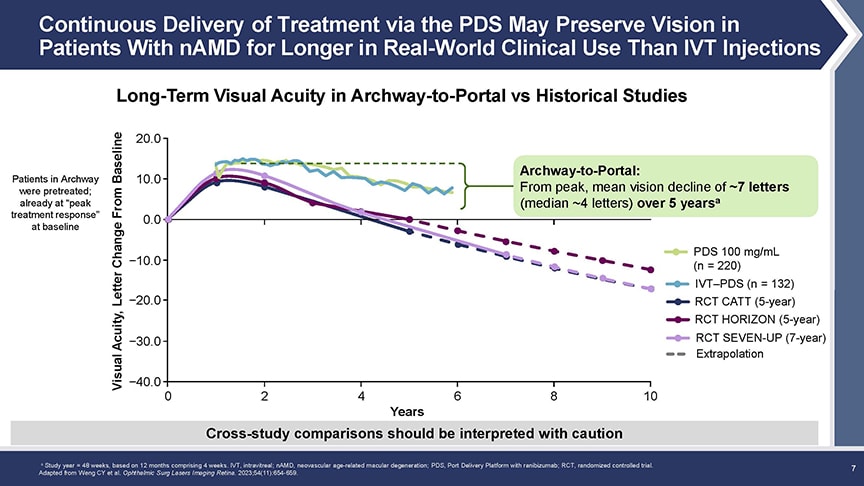
Figure 2. Over 5 years, patients in Portal lost an average of about 7 letters of visual acuity, a decline that compares favorably with the 12-letter to 17-letter losses seen in other long-term anti-VEGF studies.
Retinal thickness remained stable in both groups. In the Susvimo cohort from Archway, the average change in central subfield thickness was -1.4 µm (95% CI: -13.1, 11.1). In the IVT-to-Susvimo cohort, the reduction was -4.2 µm (95% CI: -25.7, 5.0) (Figure 3).
Both Archway and Portal had rescue criteria, so any patient whose disease was getting worse between the refills could be rescued with a ranibizumab injection. “We saw consistently 95%+ of patients could make it every 6 months without needing a rescue treatment,” said Dr. Kitchens. In a few cases with significant vision loss (>3 EDTRS lines), Dr. Kitchens reported that geographic atrophy, not surgical complications or nAMD progression, was the primary driver. “It doesn’t look like anybody had significant visual acuity loss due to severe complications from wet AMD,” he said.

Figure 3. Nearly all patients in both Archway and Portal were able to go 6 months between PDS refills without needing rescue treatment.
Another notable finding was the relatively low rate of endophthalmitis over the 5-year study period. This has been a key concern for the PDS since its launch, when an increased risk of infection was observed compared to intravitreal injections. In the current study, the overall rate of endophthalmitis was 2.6%. Notably, most of the patients had undergone implant surgery prior to a procedural update issued by Genentech in June 2020, which introduced revised surgical techniques aimed at improving safety, observed Dr. Kitchens. “We’ve seen that since that surgical update to the procedure, the risk of endophthalmitis has decreased almost threefold,” he said. “It’s reassuring to see that the rates are still fairly low, but I think with this update we’re doing even better and those rates over the long term will reflect that as we continue to follow patients.”
The cohort participating in the Portal study represents the largest group of nAMD patients followed prospectively and continuously for 5 years in a clinical trial. The study remains ongoing.
“What we’ve learned with these long-term extension studies is that when the patients get into the real world, they often get undertreated and they don't do as well,” explained Dr. Kitchens. “What this 5-year Portal study showed us, basically, was that we can do a better job when we provide continuous maintenance therapy for our patients—and one of the best ways to do that without increasing treatment burden on our patients is the port delivery system.” RP








